

影响因子2020
10.093
备受期待的2020年期刊影响因子已经发布,我们很高兴地看到 Endoscopy 的影响因子提高到了10.093。这是我们有史以来的最高影响因子! 非常感谢所有作者、审稿人和编辑的支持和最宝贵的贡献。
以下三篇是被引用次数最多的论文,它们对影响因子的提高有着重要贡献,欢迎免费阅读。
A deep neural network improves endoscopic detection of early gastric cancer without blind spots
Lianlian Wu, Wei Zhou, Xinyue Wan, Jun Zhang, Lei Shen, Shan Hu, Qianshan Ding, Ganggang Mu, Anning Yin, Xu Huang, Jun Liu, Xiaoda Jiang, Zhengqiang Wang, Yunchao Deng, Mei Liu, Rong Lin, Tingsheng Ling, Peng Li, Qi Wu, Peng Jin, Jie Chen, Honggang Yu
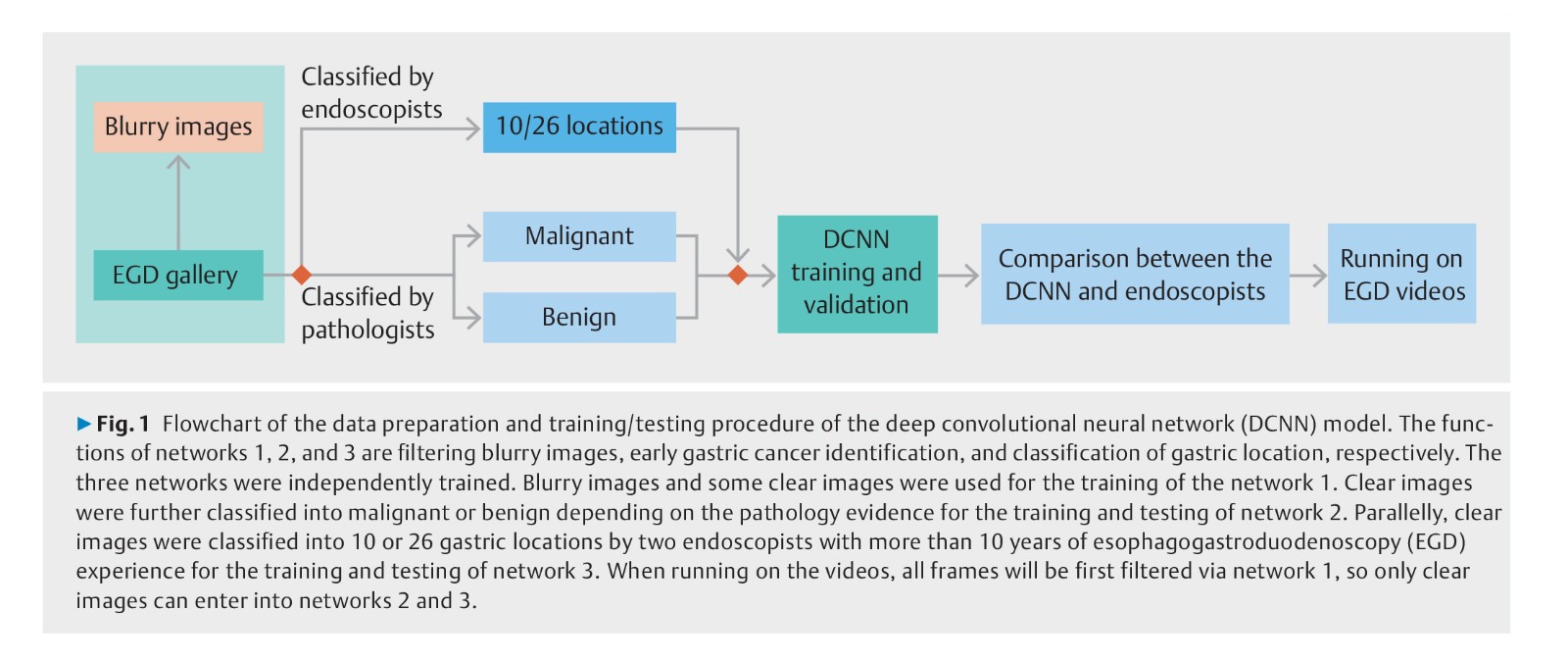
Background: Gastric cancer is the third most lethal malignancy worldwide. A novel deep convolution neural network (DCNN) to perform visual tasks has been recently developed. The aim of this study was to build a system using the DCNN to detect early gastric cancer (EGC) without blind spots during esophagogastroduodenoscopy (EGD).
Methods: 3170 gastric cancer and 5981 benign images were collected to train the DCNN to detect EGC. A total of 24549 images from different parts of stomach were collected to train the DCNN to monitor blind spots. Class activation maps were developed to automatically cover suspicious cancerous regions. A grid model for the stomach was used to indicate the existence of blind spots in unprocessed EGD videos.
Results: The DCNN identified EGC from non-malignancy with an accuracy of 92.5 %, a sensitivity of 94.0 %, a specificity of 91.0 %, a positive predictive value of 91.3 %, and a negative predictive value of 93.8 %, outperforming all levels of endoscopists. In the task of classifying gastric locations into 10 or 26 parts, the DCNN achieved an accuracy of 90 % or 65.9 %, on a par with the performance of experts. In real-time unprocessed EGD videos, the DCNN achieved automated performance for detecting EGC and monitoring blind spots.
Conclusions: Wu et al developed a system based on a DCNN to accurately detect EGC and recognize gastric locations better than endoscopists, and proactively track suspicious cancerous lesions and monitor blind spots during EGD.
Jacques Jérémie et al.
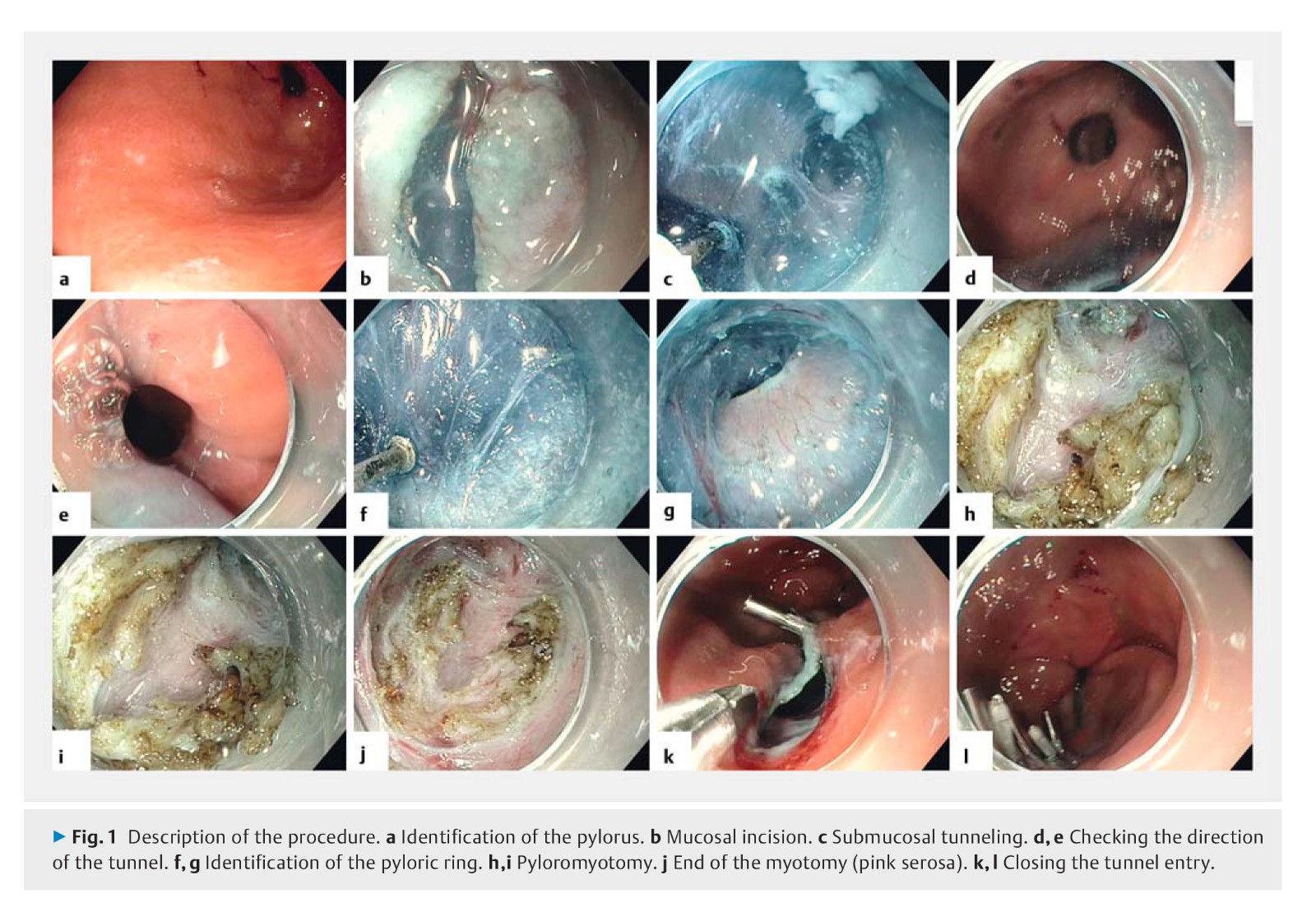
Background: Gastroparesis is a functional disorder with a variety of symptoms that is characterized by delayed gastric emptying in the absence of mechanical obstruction. A recent series of retrospective studies has demonstrated that peroral endoscopic pyloromyotomy (G-POEM) is a promising endoscopic procedure for treating patients with refractory gastroparesis. The aim of this prospective study was to evaluate the feasibility, safety, and efficacy of G-POEM.
Methods: 20 patients with refractory gastroparesis (10 diabetic and 10 nondiabetic) were prospectively included in the trial. Patients were treated by G-POEM after evaluation of pyloric function using an endoscopic functional luminal imaging probe. Clinical responses were evaluated using the Gastroparesis Cardinal Symptom Index (GCSI), and quality of life was assessed using the Patient Assessment of Upper Gastrointestinal Disorders – Quality of Life scale and the Gastrointestinal Quality of Life Index scores. Gastric emptying was measured using 4-hour scintigraphy before G-POEM and at 3 months.
Results: Feasibility of the procedure was 100 %. Compared with baseline values, G-POEM significantly improved symptoms (GCSI: 1.3 vs. 3.5; P < 0.001), quality of life, and gastric emptying (T½: 100 vs. 345 minutes, P < 0.001; %H2: 56.0 % vs. 81.5 %, P < 0.001; %H4: 15.0 % vs. 57.5 %, P = 0.003) at 3 months. The clinical success of G-POEM using the functional imaging probe inflated to 50 mL had specificity of 100 % and sensitivity of 72.2 % (P = 0.04; 95 % confidence interval 0.51 – 0.94; area under the curve 0.72) at a distensibility threshold of 9.2 mm2/mmHg.
Conclusion: G-POEM was efficacious and safe for treating refractory gastroparesis, especially in patients with low pyloric distensibility.
Barthet Marc et al.
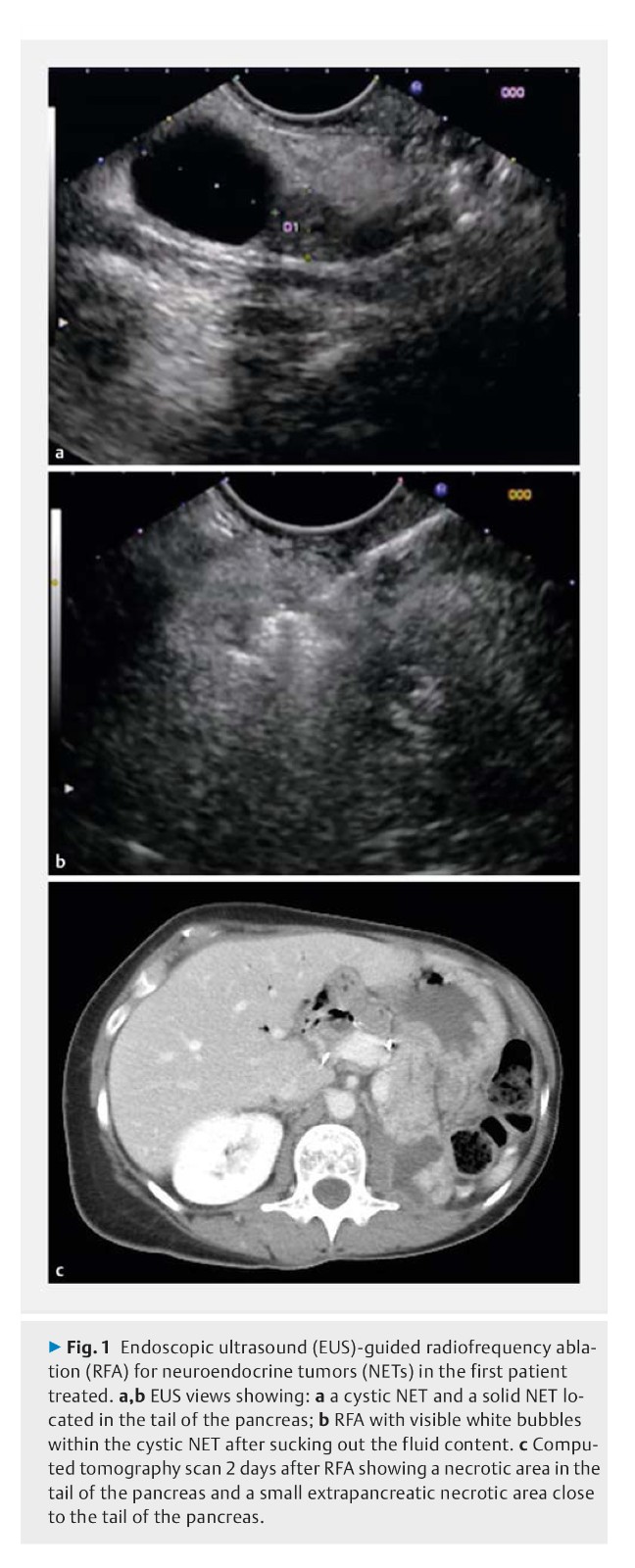
Background: Pancreatic neuroendocrine tumors (NETs) and intraductal pancreatic mucinous neoplasia (IPMN) with worrisome features are surgically managed. Endoscopic ultrasound (EUS)-guided radiofrequency ablation (RFA) has recently been developed. The safety of EUS-RFA was the primary end point of this study, its efficacy the secondary end point.
Methods: This was a prospective multicenter study that was planned to include 30 patients with a 1-year follow-up with either a NET < 2 cm or a pancreatic cystic neoplasm (PCN), either a branch duct IPMN with worrisome features or a mucinous cystadenoma (MCA). EUS-RFA was performed with an 18G RFA cooling needle.
Results: 12 patients had 14 NETs (mean size 13.1 mm, range 10 – 20 mm); 17 patients had cystic tumors (16 IPMNs, 1 MCA; mean size 28 mm, range 9 – 60 mm). Overall three adverse events occurred (10 %), two of these in the first two patients (one pancreatitis, one small-bowel perforation). After these initial patients, modifications in the protocol resulted in a decrease in complications (3.5 %), with one patient having a pancreatic ductal stenosis. Among the 14 NETs, at 1-year follow-up 12 had completely disappeared (86 % tumor resolution), with three patients having a delayed response. Among the 17 PCNs, at 12 months, there were 11 complete disappearances and one diameter that decreased by > 50 % (significant response rate 71 %). All 12 mural nodules showed complete resolution.
Conclusions: EUS-RFA of pancreatic NETs or PCNs is safe with a 10 % complication rate, which can be decreased by improved prophylaxis for the procedure.
阅读本刊更多论文,请点击这里。
