
Thieme Chemistry
Keep Track of the Latest Content
欢迎您阅读SYNTHESIS、SYNLETT、SynOpen和Organic Materials的精选论文,并通过SYNFORM深入了解化学界最新动态。
Our Highlighted Articles and Specials
Synthesis
Synthesis of Chiral Amines by C–C Bond Formation with Photoredox Catalysis
Stephen T. J. Cullen, Gregory K. Friestad
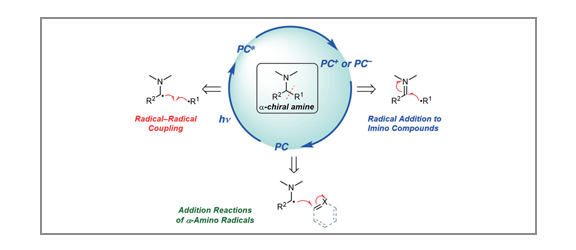
Chiral amines are key substructures of biologically active natural products and drug candidates. The advent of photoredox catalysis has changed the way synthetic chemists think about building these substructures, opening new pathways that were previously unavailable. New developments in this area are reviewed, with an emphasis on C–C bond constructions involving radical intermediates generated through photoredox processes.
Synlett
Cooperative Hydrogen Atom Transfer: From Theory to Applications
Padmanabha V. Kattamuri, Julian G. West
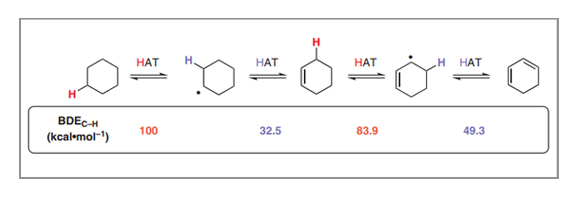
Hydrogen atom transfer (HAT) is one of the fundamental transformations of organic chemistry, allowing the interconversion of open- and closed-shell species through the concerted movement of a proton and an electron. Although the value of this transformation is well appreciated in isolation, with it being used for homolytic C–H activation via abstractive HAT and radical reduction via donative HAT, cooperative HAT (cHAT) reactions, in which two hydrogen atoms are removed or donated to vicinal reaction centers in succession through radical intermediates, are comparatively unknown outside of the mechanism of desaturase enzymes. This tandem reaction scheme has important ramifications in the thermochemistry of each HAT, with the bond dissociation energy (BDE) of the C–H bond adjacent to the radical center being significantly lowered relative to that of the parent alkane, allowing each HAT to be performed by different species. Herein, we discuss the thermodynamic basis of this bond strength differential in cHAT and demonstrate its use as a design principle in organic chemistry for both dehydrogenative (application 1) and hydrogenative (application 2) reactions. We hope that this overview will highlight the exciting reactivity that is possible with cHAT and inspire further developments with this mechanistic approach.
Organic Materials
P. Chidchob, S. A. H. Jansen, S. C. J. Meskers, E. Weyandt, N. P. van Leest, B. de Bruin, A. R. A. Palmans, G. Vantomme, E. W. Meijer
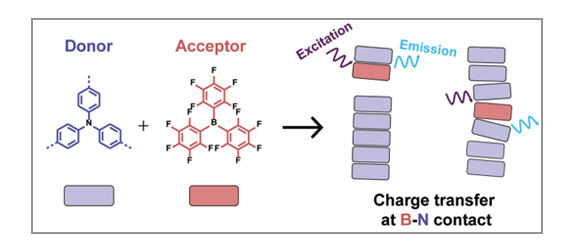
The introduction of a chemical additive to supramolecular polymers holds high potential in the development of new structures and functions. In this regard, various donor- and acceptor-based molecules have been applied in the design of these noncovalent polymers. However, the incorporation of boron–nitrogen frustrated Lewis pairs in such architectures is still rare despite their many intriguing properties in catalysis and materials science. The limited choices of suitable boron derivatives represent one of the main limitations for the advancement in this direction. Here, we examine the use of the commercially available tris(pentafluorophenyl)borane with various triphenylamine derivatives to create supramolecular B–N charge transfer systems. Our results highlight the importance of a proper balance between the donor/acceptor strength and the driving force for supramolecular polymerization to achieve stable, long-range ordered B–N systems. Detailed analyses using electron paramagnetic resonance and optical spectroscopy suggest that tris(pentafluorophenyl)borane displays complex behavior with the amide-based triphenylamine supramolecular polymers and may interact in dimers or larger chiral aggregates, depending on the specific structure of the triphenylamines.
SynOpen
Valerio Zullo, Antonella Petri, Anna Iuliano
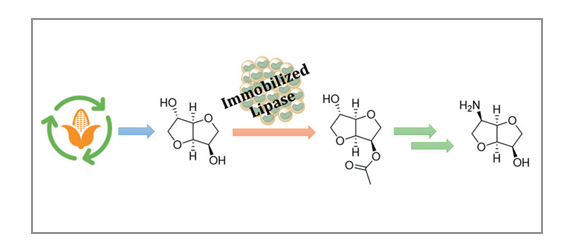
The synthesis of 6-aminoisomannide is easily achieved starting from the renewable, inexpensive, and commercially available isosorbide in 66% overall yield. A biocatalyzed highly regioselective acetylation of the 3-endo hydroxyl group of isosorbide was followed by the stereospecific interconversion of the 6-exo hydroxyl group into an azido group, through reaction with trifluoromethanesulfonic anhydride, followed by nucleophilic displacement of the triflate group by sodium azide. Finally, reduction of the azido group and deacetylation of the 3-hydroxy group were performed in one pot using LiAlH4.
Synform
SYNFORM regularly meets young up-and-coming researchers who are performingexceptionally well in the arena of organic chemistry and related fields of research, in order to introduce themto the readership. This Young Career Focus presents Prof. Dr. Alexey Sukhorukov (N. D. Zelinsky Institute ofOrganic Chemistry, and D. Mendeleev University of Chemical Technology, Russian Federation).
